1197
Views & Citations197
Likes & Shares
Kigelia africana has been used by population as an herbal medicine to treat several diseases, such as inflammatory disorders, infectious diseases and malaria. Thus, this study aimed to characterize the K. africana fruit extract (KAFE) by thermogravimetry (TG), differential thermal analysis (DTA) and pyrolysis coupled to gas chromatography and mass spectrometry (Pyr-GC/MS) and analysis of its anti-inflammatory and schistosomicidal activities. The extracts were dried by spray dryer at 160°C and rotary evaporator at 40°C. All samples were submitted to dynamic TG in two different atmospheres (nitrogen and air), TG-isothermal in the air and DTA in the nitrogen. The molecules degradable at 250°C and 500°C were identified by Pyr-GC/MS. The dynamic TG curves showed six decomposition steps for all samples in the nitrogen and air. The extract (S. dryer) had greater loss of the total mass, take less time for degradation of 5% of the mass at 30°C, showed lower enthalpy and activation energy than extract (R. evaporator) were identified 47 different molecules in all samples. The extracts showed the anti-inflammatory effect acting against protein denaturation; however, for schistosomicidal activity it was not active.
Keywords: Kigelia africana extracts, Thermo analytical characterization, Anti-inflammatory, Schistosomicidal activities
Abbreviations: A: Frequency factor; KAFE: Kigelia africana Fruit Extract; DTA: Differential Thermal Analysis; Ea: Activation Energy; EI: Electron Impact; iNOS: inducible Nitric Oxide Synthase; K: Rate Constant; LPS: Lipopolysaccharide; m/z: Mass-to-Charge Ratio; n: Reaction Order; NO: Nitric Oxide; Pyr-GC/MS: Pyrolysis Coupled to Gas Chromatography Interfaced with Mass Spectrometry; R: Gas Constant (8.314 J K-1 mol-1); RT: Retention Time; T: Temperature; TG: Thermogravimetry
In the technological development of phytotherapeutic drugs, the extracts have been submitted to several drying techniques to guarantee the physical and chemical stability of the intermediate product [13]. Spray dryer and rotary evaporator are commonly used techniques for drying vegetable extracts, because they offer a dry product suitable for technological production of phytotherapeutic drugs [14-16].
The thermogravimetry (TG) and differential thermal analysis (DTA) are the most commonly used thermoanalytical techniques to characterize the dry extracts as raw material for technological production of phytotherapeutic drugs [17]. These techniques analyze the thermal decomposition profile and kinetic parameters, such as reaction order (n), activation energy (Ea), frequency factor (A) and degradation constant [18]. Pyrolysis coupled to gas chromatography and mass spectrometry (Pyr-GC/MS) has been used together with TG and DTA to identify molecular fragments degradable resulting from the temperature processes [19].
In our best knowledge, there is no available information in the literature about physicochemical quality evaluation of KAFE as raw material for the technological development of phytotherapeutic drugs. Thus, in the present research study we characterized the K. africana fruit extract by thermogravimetry (TG), differential thermal analysis (DTA) and pyrolysis coupled to gas chromatography and mass spectrometry (Pyr-GC/MS) and analysis of its anti-inflammatory and schistosomicidal activities.
MATERIALS AND METHOD
Plant material and powder preparation
The fruits of K. africana was collected in Buzi-Sofala/Mozambique (19°34’08” and 20°33’09” S/34°46’57” and 33°53’07” W), in January, 2018. The plant material was dehydrated in an oven at 40°C and was subsequently milled and pulverized. The material obtained by each mesh was stored in a frosted polyethylene container, properly sealed and protected from light and humidity at room temperature.
Preparation of extract
The extraction was done in two ways namely, maceration and supercritical fluid extraction.
Maceration: 200 g of plant powder was subjected to dynamic maceration in 2000 mL hydroethanolic solution (ethanol 70%) for 6 h at 1100 rpm. The mixture was filtered using dry cotton followed by filter paper. In order to determine the dry residue, the fluid extract was placed in a drying oven at 105°C for 2 h and then cooled in a desiccator. The spray-dried filtrate was prepared from a suspension containing 20% of colloidal silicon dioxide. During the atomization procedure, the mixture was mixed with a magnetic stirring bar. The drying temperature was 160°C and the pump flow was 8 mL min-1. The dry extract was stored at room temperature in plastic containers with lid, to minimize possible changes in the material, such as agglomeration caused by water absorption and oxidation, until further use. The product was named extract (S. dryer).
Supercritical fluid extraction: Two hundred grams of K. africana fruit powder was placed in a reactor model 4848B. Then, 2000 mL of hydroethanolic solution (EtOH 70%) was placed in the same reactor. 35 bar of CO2 was applied to the reactor. The reaction occurred at 40°C for 5 h at 747 rpm. The extract was dried in rotary evaporator at 40°C. The product was stored at room temperature in plastic containers with lid and named extract (R. evaporator).
Qualitative analysis on phytochemical constituents
The preliminary phytochemical studies on the K. africana extracts were conducted as per the standard procedures [20].
TG-dynamic: The dynamic TG curves were acquired using a Shimadzu thermobalance, model TGA-60, using an alumina crucible. Rising temperature experiments were conducted in the temperature range 40-900°C, at 10°C min-1 in the nitrogen atmosphere (50 mL min-1 flow) and air atmosphere (20 mL min-1 flow), with a sample mass of 5.000 ± 0.002 mg, in both tested atmospheres. The data were analyzed using the TASYS (TA-60) and Origin Pro 8.0.
TG-isothermal: The TG-isothermal curves were carried out over 240 min in air atmosphere at different temperatures: 160, 170, 180, 190 and 200°C, according to TG-dynamic profiles. The data were analyzed using the TASYS (TA-60) and Origin Pro 8.0. The degradation constant was calculated using the Arrhenius equation. While, the degradation time of 5% of the mass at 30°C was calculated based on the equation of the zero-order.
Kinetic parameters
The degradation kinetic parameters, namely reaction order (n), activation energy (Ea) and frequency factor (A) were determined by TG-isothermal, using the Arrhenius equation (lnK=lnA – Ea/RT).
Differential thermal analysis
DTA curves of the samples were recorded using a Shimadzu differential thermal analyzer model DTA-50 in the nitrogen (50 mL min-1 flow) at 10°C min-1, up to the temperature of 900°C. The samples mass was 5.000 ± 0.003 mg, which was analyzed by its typical transition phases, using the TA-60WS software (Shimadzu, Japan). The DTA curves were plotted using Origin Pro 8.0.
Pyrolysis coupled to gas chromatography and mass spectrometry
Pyr-GC/MS was used together with TG and DTA to identify the molecular fragments degradable resulting from the temperature processes. A capillary column with stationary phase phenyl/dimethyl polysiloxane (5:95) (with 30 m length, 0.25 mm internal diameter and 0.25 µm particle sizes) was used. The gas chromatograph was interfaced with the mass spectrometer, which was configured to scan a mass range from m/z 50 to 450. Helium was used as a gas carrier at a flow rate of 1.7 mL min-1 and a split ratio of 1:5. Ionization was effected by electron impact (EI) at 70 eV. The chromatographic run was performed using a temperature ramp at rate 10°C min-1, starting at 70 up to 300°C, for 28 min. A fraction of the KAFE samples in the platinum crucible was introduced in the pyrolyzer preheated at temperatures of 250 and 500°C, separately for each experiment. These temperatures were selected according to the dynamic TG curves of the samples under a nitrogen atmosphere. The molecular fragments were identified through mass-charge ratio using GCMS Real Time Analysis and GCMS Postrun Analysis software. The identification of the compounds was made by comparing their mass spectra with the Wiley/NBS library reference data (6th Edn for Class-5000).
Anti-inflammatory activity
The reaction mixture (5 mL) consisted of 0.2 mL of egg albumin (from fresh hen’s egg), 2.8 mL of phosphate buffered saline (PBS, pH 6.4) and 2 mL of varying concentrations of KAFE so that final concentrations become 100, 250, 500, 750, 1000 µg/mL. Similar volume of double-distilled water served as control. Then the mixtures were incubated at (37 ± 2)°C in an incubator for 15 min and then heated at 70°C for 5 min. After cooling, their absorbance was measured at 660 nm (SHIMADZU, UV 1800) by using vehicle as blank. Diclofenac sodium (100 µg/mL) was used as reference drug and treated similarly for determination of absorbance. The inhibition percentage of protein denaturation was calculated by using the following formula:
%inhibition = Absorbance of test / Absorbance of control * 100
Schistosomicidal activity
S. mansoni mates were placed in 24-well cell culture plates (one pair per well) containing 1 mL of RPMI 1640 (Roswell Park Memorial Institute Medium), amphotericin (2 μg/mL), penicillin (200 U/mL), streptomycin (200 μg/mL) and 10% of fetal bovine serum (FBS). 100 µg/mL of extract was added to the culture medium with 1.5% of dimethylsulfoxide (DMSO) (negative control) and 1500 ng/mL of praziquantel (PZQ) (positive control). The parasites were maintained for 96 h at 37°C in CO2 atmosphere (5% CO2). The schistosomicidal activity of the extract was evaluated through the analysis of motor skills and reproductive capacity. The observations were made through the inverted microscope, and the first observation was made two hours after the addition of the extract. Subsequent observations were made at 24 h intervals for four days. Mortality was established by the total absence of movements. The reproductive capacity was evaluated by mating and oviposition. The eggs were counted at the end of the experiment using a stereomicroscope.
RESULTS
Qualitative analysis on phytochemical constituents
Qualitative analysis carried out on KAFE showed the presence of various phytochemical constituents and the results are shown in Table 1. Similar results were verified by Abdulkadir et al. [21].
TG-dynamic
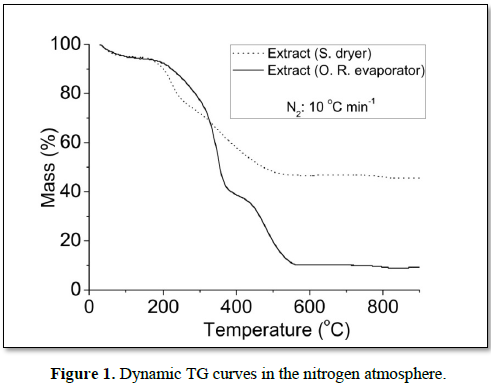
In the nitrogen atmosphere, the dynamic TG curves showed five decomposition steps for all samples (Figure 1). The first decomposition step of extract (S. dryer) occurred in the range of 76.0-121.0°C, referring to the volatiles, mainly water, corresponding to 5.92% and the main step represents the macro and micro compounds degradations occurring in the temperature ranges of 321.6-473.7, having lost 19.37% of the mass. For dry extract in rotary evaporator the first decomposition step occurred in the range of 45.0-119.0°C, corresponding to 6.43% and the main step of mass loss was recorded between 322.4 and 367.5, having lost 33.16%. The total mass losses during all decomposition steps were 49.72 and 87.31% for extract (S. dryer) and extract (R. evaporator), respectively. The extract (S. dryer) showed greater amount of residue than extract (R. evaporator) (Figure 1). Suggesting that this extract presents greater physical stability [14,15], probably due to its higher level of dehydration than extract dried in oven-drying.
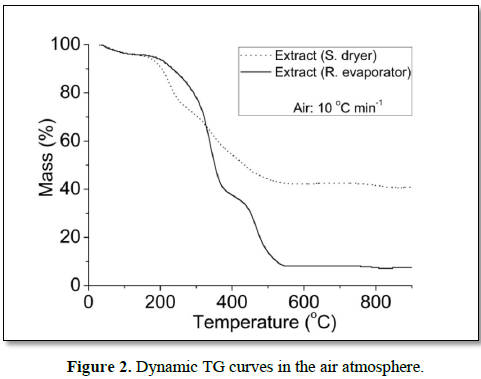
In the air, the dynamic TG curves showed six decomposition steps for all samples (Figure 2). For extract (S. dryer), the temperature range of the main step occurred between 343.7 and 456.2°C, having lost 21.94% of the mass, and for (R. evaporator), the main step of mass loss occurred between 298.7 and 363.8 having lost 36.78%. The dynamic TG curves of extract (S. dryer), showed higher thermal stability, because showed greater amount of residue and lower total mass loss than extract (R. evaporator). Similar results were observed in dynamic TG curves under a nitrogen atmosphere.
TG-isothermal
The degradation time of 5% of the mass at 30oC and thermal degradation constant at different temperatures, for plant drug and extract samples are shown in (Table 2). We have observed that the greatest mass loss occurred at high temperatures for all evaluated samples. Which means that the majority part of the substances present in K. africana extracts degrade at high temperatures. The thermal degradation constant determined by Arrhenius equation evidenced larger values for extract (R. evaporator) as compared to the dry extract through the spray dryer. In this context, extract (R. evaporator) is more unstable than extract (S. dryer). The result is corroborated through the values that determine the validity of the raw material, in which the required time for the degradation of 5% of the sample mass at room temperature (30°C) was 1.136 and 0.768 years for extract (S. dryer) and extract (R. evaporator) respectively.
Kinetic parameters
The results obtained by Arrhenius equation application showed the zero order for all samples evaluated by the derived method (Table 3). The activation energy values (Ea) were different between extract (S. dryer) and extract (R. evaporator) in the air atmosphere. The dry extract through spray dryer showed lower activation energy than dry extract via rotary evaporator. In this case, the extract (S. dryer) are more available to interact with the heat and required less energy to initiate its chemical degradation process.
Differential thermal analysis
DTA curves showed two exothermic peaks for all samples, described in terms of temperature and enthalpy (Table 4). The peaks provide differences in terms of released heat between the samples. In all samples, the second peak occurred near to 500°C and presented higher values of enthalpy than the first. Were observed that extract (S. dryer) presented lower enthalpy comparing with extract (R. evaporator). According to the data analysis is possible to specify each sample considering the released heat.
Pyrolysis coupled to gas chromatography interfaced with mass spectrometry
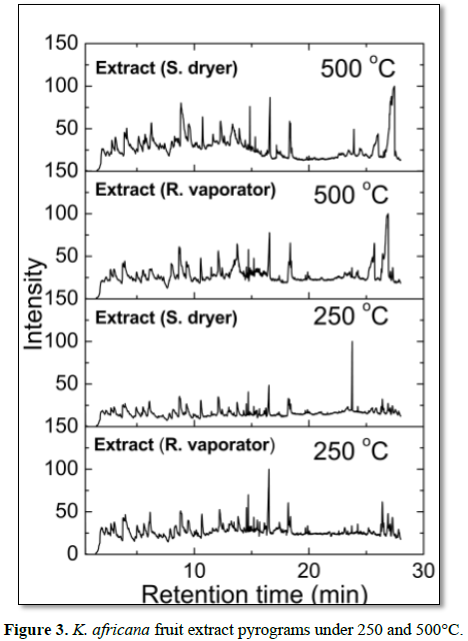
The molecules identified as degradation products through retention time when evaluated semi-quantitatively in relation to their peak areas demonstrate percentage variations between different samples for all measured temperatures (Figure 3). The main molecules released during the stages of thermal degradation and its relative area percentages are shown in (Table 5). To calculate the percentage of the relative area of peaks matching to molecules was considered the sum of the areas of all peaks analyzed as 100%. In all extract samples, we identified 47 different molecular fragments. Of these, the main compounds identified were 2-furanmethanol (C5H6O2) at 3.96 min, phenol-2-methoxy (C7H8O2) at 5.52 min, limonene (C10H16) at 6.10 min, oleic acid (C18H34O2) at 16.06 min, palmitic acid (C16H32O2) at 16.54 min, linoleic acid (C18H34O2) at 17.69 min, 8,11,14-eicosatrienoic acid (C20H34O2) at 18.37 min, stearic acid (C18H36O2) at 21.45 min, flavone 4-OH, 5-OH, 7-di-O-glucoside (C27H30O15) at 26.38 min, at the temperature of 500oC (Figure 3). At 250oC were identified 2,6 dimethoxyphenol (C8H10O3) at 9.46 min, 2,6, oleic acid (C18H34O2) at 16.05 min, linoleic acid (C18H34O2) at 17.67 min, palmitic acid (C16H32O2) at 20.41 min and vitamin E (C29H50O2) at 27.23 min (Figure 3). These molecules remain intact in lower temperature to higher, however, the linoleic acid, oleic acid and palmitic acid were observed in all samples at 250 and 500°C.
Anti-inflammatory activity
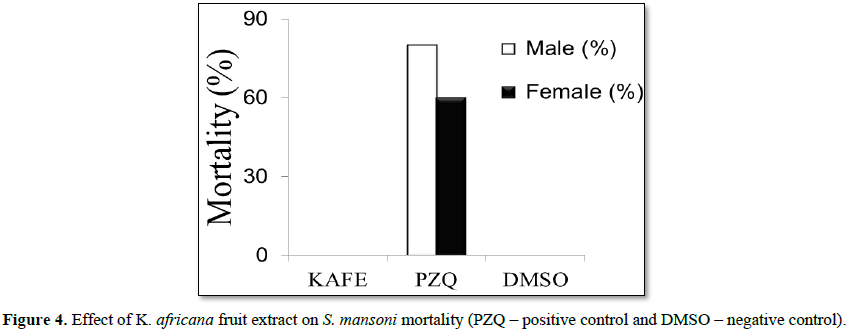
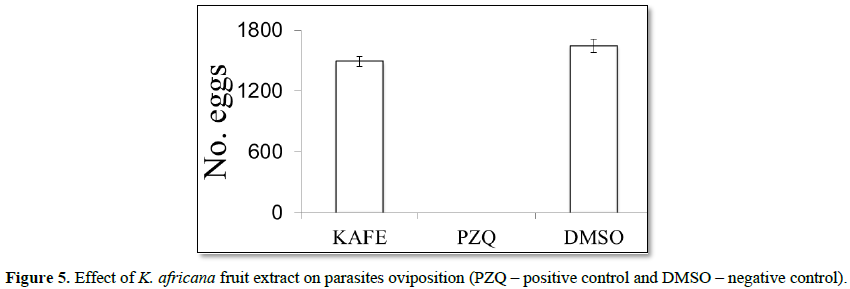
Denaturation of tissue proteins is one of the well-documented causes of inflammatory diseases. Production of auto antigens in certain arthritic diseases may be due to denaturation of proteins in vivo [22,23]. Agents that can prevent protein denaturation therefore, would be worthwhile for anti-inflammatory drug development [24].Thus the in vitro anti-inflammatory effect of KAFE was evaluated against denaturation of egg albumin and the results are shown in Table 6. The present findings exhibited a concentration dependent inhibition of protein (albumin) denaturation by KAFE throughout the concentration range of 100 to 1000 µg/mL. Diclofenac sodium (100 µg/mL) was used as reference drug which also exhibited concentration dependent inhibition of protein denaturation. The effect of extract (1000 µg/mL) was significantly greater (p<0.05) than diclofenac sodium (100 µg/mL). Similar results were observed by Umapathy et al. [22]. It has been reported that one of the features of several non-steroidal anti-inflammatory drugs is their ability to stabilize (prevent denaturation) heat treated albumin at the physiological pH (pH=6.2-6.5) [25]. The major constituents of KAFE are polyphenolic compounds like flavonoids and tannins, because these compounds have been affecting the function of T-cell, mast cell and enzyme systems involved in immune response and inflammatory process generation, such as inhibition of NF-kB activation, iNOS expression and COXs [26], however, the effect may be due to the synergistic effect rather than single constituent. K. africana extract had no effect on mortality or oviposition in S. mansoni mating. In PZQ (positive control group), the mortality was 80% for male and 60% for female, and oviposition inhibition was 100%. DMSO (negative control) did not kill the parasites and did not inhibit oviposition (Figures 4 and 5).
CONCLUSION
A Through the thermal analysis we have recorded that the mass loss of K. africana extracts depends on the physicochemical characteristics of the sample. The dry extract through spray dryer showed higher thermal stability than extract (R. evaporator) and take longer time for degradation of 5% of the mass at room temperature. For technological production of phytotherapics, the extract (S. dyer) showed better properties than extract (R. evaporator). The extracts showed the anti-inflammatory effect acting against protein denaturation; however, for schistosomicidal activity it was not active. These results suggested that K. africana extract dried through the spray dryer could be potential candidates for the technological production of phyto therapeutic drugs.
ACKNOWLEDGMENT
The authors are grateful to Student Program-Postgraduate Agreement (PEC-PG) of the CAPES/CNPq – Brazil, for financial support.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
1. Grace OM, Light ME, Lindsey KI, Mulholland DA, Van Staden J, et al. (2002) Anti-bacterial activity and isolation of active compounds from fruit of the traditional African medicinal tree Kigelia africana. S Afr J Bot 68: 220-222.
2. Araújo KRM, Kerntopf MR, Oliveira DR, Menezes IRA, Brito Júnior FE, et al. (2012) Medicinal plants in the treatment of respiratory diseases in childhood: A view from popular knowledge. Rev Rene 13: 659-666.
3. Azu OO (2013) The sausage plant (Kigelia africana): Have we finally discovered a male sperm booster? J Med Plant Res 7: 903-910.
4. Elhasan GO, Omer SM, Khan MU, Abdul Kareem AKM, Khalilullah H, et al. (2014) Kigelia africana: An Ephemeral glance. Int J Pharmacol Pharm Sci 2: 20-25.
5. Binutu OA, Adesogan KE, Okogun JI (1996) Antibacterial and antifungal compounds from Kigelia pinnata. Planta Med 62: 352-353.
6. Owolabi OJ, Omogbai EKI, Obasuyi O (2007) Antifungal and antibacterial activities of the ethanolic and aqueous extract of Kigelia africana (Bignoniaceae) stem bark. Afr J Biotechnol 6: 1677-1680.
7. Agyare C, Dwobeng AS, Agyepong N, Boakye YD, Mensah KB, et al. (2013) Antimicrobial, antioxidant and wound healing properties of Kigelia africana (Lam.) Beneth. and Strophanthus hispidus D C. Adv Pharmacol Sci.
8. Azu OO, Duru FIO, Osinubi AA, Noronha CC, Elesha SO, et al. (2010) Preliminary study on the antioxidant effect of Kigelia africana fruit extract (Bignoniacieae) in male Sprague-Dawley rats. Afr J Biotechnol 9: 1374-1381.
9. Azuine MA, Ibrahim K, Enwerem NM, Wambebe C, Kolodziej H, et al. (1997) Protective role of Kigelia africana fruits against benzo[a] pyrene-induced fore stomach tumourigenesis in mice and against albumen-induced inflammation in rats. Pharm Pharmacol Lett 7: 67-70.
10. Picerno P, Autore G, Marzocco S, Meloni M, Sanogo R, et al. (2005) Anti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J Nat Prod 68: 1610-1616.
11. Owolabi OJ, Omogbai EKI (2007) Analgesic and anti-inflammatory activities of the ethanolic stem bark extract of Kigelia africana (Bignoniaceae). Afr J Biotechnol 6: 582-585.
12. Atawodi SEO, Olowoniyi OD (2015) Pharmacological and therapeutic activities of Kigelia africana (Lam.) Benth. Annu Res Rev Biol 5: 1-17.
13. List PH, Schimidt PC (1989) Phyto pharmaceutical technology. Boca Raton: CRC.
14. Souza KCB, Petrovick PR, Bassani VL, Ortega GG (2000) The adjuvants aerosil 200 and gelita-sol-p influence on the technological characteristics of spray-dried powders from Passiflora edulis var. flavi carpa. Drug Dev Ind Pharm 26: 331-336.
15. Oliveira PC, Conceição EC, Oliveira PA, Nascimento MV, Costa EA, et al. (2012) Obtaining a dry extract of Pterodon emarginatus (Fabaceae) fruits by spray-drying. J Pharm Res 5: 641-645.
16. Silva RMF, Gomes TCBL, Albuquerque MM, Silva Junior JOC, Barbosa WLR, et al. (2012) Approach on different drying processes employed for obtaining dry extracts from medicinal plants. Rev Bras Pl Med Botucatu 14: 103-109.
17. Wesołowski M, Konieczynski P (2003) Thermo analytical, chemical and principal component analysis of plant drugs. Int J Pharm 262: 29-37.
18. Corrigan OI (1993) Thermal analysis of spray dried products. Thermochimica Acta Irlanda 248: 245-258.
19. Wang L, Wang C, Pan Z, Sun Y, Zhu X, et al. (2011) Application of pyrolysis-gas chromatography and hierarchical cluster analysis to the discrimination of the Chinese traditional medicine Dendrobium candidum Wall. ex Lindl. J Anal Appl Pyrol 90: 13-17.
20. Krishnaiah D, Devi T, Bono A, Sarbatly R (2009) Studies on phytochemical constituents of six Malaysian medicinal plants. J Med Plants Res 3: 67-72.
21. Abdulkadir MN, Adedokun A, John E (2015) Phytochemical composition and antimicrobial evaluation of Kigelia africana Lam. Asian J Plant Sci Res 5: 14-17.
22. Umapathy E, Ndebia EJ, Meeme A, Adam B, Menziwa P, et al. (2010) An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J Med Plants Res 4: 789-795.
23. Opie EL (1962) On the relation of necrosis and inflammation to denaturation of proteins. J Exp Med 115: 597-608.
24. Anson ML, Mirsky AE (1932) The effect of denaturation on the viscosity of protein systems. Gen Physiol 15: 341-350.
25. García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, et al. (2007) The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein and down-regulation of the nuclear factor kappa B pathway in Chang liver cells. Eur J Pharmacol 557: 221-229.
26. Williams LAD, O’Connar A, Latore L, Dennis O, Ringer S, et al. (2008) The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med J 57: 327-331.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Astronomy and Space Research